The New Survey Process Is Here… What You Need To Know
Effective November 28, 2017, the new long term care survey process began. This new process combined the strengths from both the traditional survey process as well as the Quality Indicator Survey (QIS) to form the new process that is intended to identify concerns in a more efficient manner. Similar to the QIS survey process, the entire new long term care survey process will now be computerized.
3 Parts of the Survey Process:
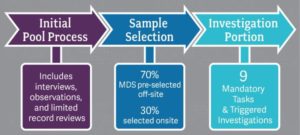
Surveyors begin preparation off-site including accessing MDS information and independently reviewing facility history. Once on-site, there is no formal tour process; surveyors will immediately go to their assigned area and begin screening residents to identify approximately eight residents, per surveyor, to include in the initial pool process. During the initial pool process, surveyors will interview selected residents, or their representative if the resident is determined to be non-interviewable, conduct observations, and complete a limited record review.
This initial pool process is expected to take 8-10 hours and focuses on the following care areas:
Choices
Activities
Dignity
Abuse
Resident-to-resident interaction
Privacy
Accommodation of Needs (physical)
Personal Funds
Personal Property
Sufficient Staffing
Participation in Care planning
Community Discharge
Environment
Food
Dental
Nutrition
Hydration
Tube Feeding
Vision and Hearing
ADLs
ADL Decline
Catheter
Insulin or Blood Thinner
Respiratory Infection
Infections (not UTI or resp.)
Hospitalizations
UTI
Falls
Pain
Pressure Ulcers
Skin Conditions (non-pressure related)
Limited ROM
Rehab
Dialysis
B&B incontinence
Constipation/diarrhea
Smoking
Hospice
Other Concerns
18 Month Moratorium Penalties:
CMS has announced that skilled nursing care providers will have 18 months to adapt to some of the new requirements of participation (RoP) without facing penalties, although surveyors will still issue citations for deficiencies under these tags. F-Tags included in the 18-month moratorium of CMPs.
- F655 (Baseline Care Plan); §483.21(a)(1)-(a)(3)
- F740 (Behavioral Health Services); §483.40F741 (Sufficient/Competent Direct Care/Access Staff-Behavioral Health); §483.40(a)(1)-(a)(2)
- F758 (Psychotropic Medications) related to PRN Limitations §483.45I(3)-I(5)
- F838 (Facility Assessment); §483.70I
- F881 (Antibiotic Stewardship Program); §483.80(a)(3)
- F865 (QAPI Program & Plan) related to development of the QAPI Plan; §483.75(a)(2) and,
- F926 (Smoking Policies). §483.90(i)(5)
Come January, CMS will use only the last two survey cycles’ worth of standard survey and complaint data to calculate Five-Star Health Inspection domain.
- Approximately 20% of SNFs are expected to see a change in their Health Inspection domain
- Approximately 15% of SNFs are expected to see a change in Overall Five-Star rating
Remember, this does not mean that you will not receive the tags listed above that will eventually be calculated into the Nursing Home Compare Five-Star Rating System.
The temporary moratorium on imposing CERTAIN enforcement remedies for specific Phase 2 requirements will last 18 months. This time frame will be used to educate facilities about Phase 2 requirements. Furthermore, the survey findings of facilities surveyed under the new LTC survey process will be published on Nursing Home Compare, but will not be incorporated into calculations for the Five-Star Quality Rating System for 12 months.
Contact your Proactive Consultant for assistance with survey preparation including facility assessment help, phase 1-3 RoP preparation, and mock survey.
